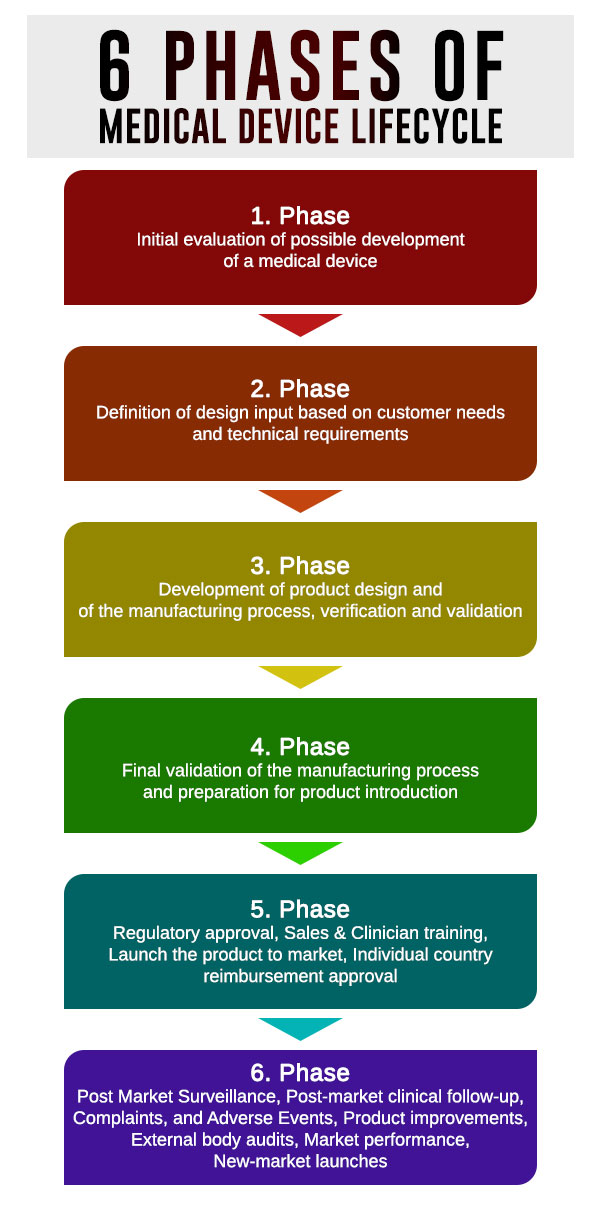
Medical Device Product Family Definition . For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any. Web organizations should develop and maintain a medical device file for each product type or device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web iso 13485 requires a medical device file for each medical device type or medical device family. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for.
from www.vrogue.co
Web iso 13485 requires a medical device file for each medical device type or medical device family. Web organizations should develop and maintain a medical device file for each product type or device family. For the purposes of this regulation, the following definitions apply: Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. (1) ‘medical device’ means any. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing.
Understanding The 7 Phases Of Medical Device Developm vrogue.co
Medical Device Product Family Definition (1) ‘medical device’ means any. (1) ‘medical device’ means any. Web iso 13485 requires a medical device file for each medical device type or medical device family. Web organizations should develop and maintain a medical device file for each product type or device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. For the purposes of this regulation, the following definitions apply:
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Product Family Definition (1) ‘medical device’ means any. Web organizations should develop and maintain a medical device file for each product type or device family. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. For the. Medical Device Product Family Definition.
From enterprisepeak.com
Medical Devices from Concept to Commercialization Enterprise Peak Medical Device Product Family Definition For the purposes of this regulation, the following definitions apply: Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. Web iso 13485 requires a medical device file for each medical. Medical Device Product Family Definition.
From perfectusbiomed.com
Supporting you on your medical device product development journey Medical Device Product Family Definition Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. (1) ‘medical device’ means any. Web iso 13485 requires a medical device file for each medical device type or medical device family. Web when grouping devices together to create a family of products for technical files, rather than have a technical file. Medical Device Product Family Definition.
From company.biomeder.com
Services Consulting — BIOMEDER Medical Device Product Family Definition Web iso 13485 requires a medical device file for each medical device type or medical device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. For the purposes of this regulation, the following definitions apply: Web when grouping devices together to create a family of products for technical files, rather. Medical Device Product Family Definition.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Product Family Definition Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web iso 13485 requires a medical device file for each medical device type or medical device family. (1) ‘medical device’ means any. Web when. Medical Device Product Family Definition.
From www.oxfordcc.co.uk
Software as a Medical Device Oxford Computer Consultants Medical Device Product Family Definition Web iso 13485 requires a medical device file for each medical device type or medical device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. For the purposes of this regulation, the following definitions apply: Web organizations should develop and maintain a medical device file for each product type or. Medical Device Product Family Definition.
From www.vrogue.co
The Process Phases Of Medical Device Development Down vrogue.co Medical Device Product Family Definition Web organizations should develop and maintain a medical device file for each product type or device family. For the purposes of this regulation, the following definitions apply: Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. Web manufacturers faced with testing multiple devices with common packaging systems to. Medical Device Product Family Definition.
From waddellgrp.com
So you want to make a Medical Device . . . Medical Project Management Medical Device Product Family Definition For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. Web iso 13485 requires a medical device file for each medical device type or medical device family. Web the food and drug administration (fda). Medical Device Product Family Definition.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Product Family Definition Web iso 13485 requires a medical device file for each medical device type or medical device family. (1) ‘medical device’ means any. Web organizations should develop and maintain a medical device file for each product type or device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web when grouping. Medical Device Product Family Definition.
From learn.marsdd.com
Healthcare product development―Step 1 Classify your healthcare product Medical Device Product Family Definition (1) ‘medical device’ means any. For the purposes of this regulation, the following definitions apply: Web organizations should develop and maintain a medical device file for each product type or device family. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web when grouping devices together to create a family of. Medical Device Product Family Definition.
From qbdgroup.com
What is a medical device? Key global definitions and regulations Medical Device Product Family Definition (1) ‘medical device’ means any. Web iso 13485 requires a medical device file for each medical device type or medical device family. For the purposes of this regulation, the following definitions apply: Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web the food and drug administration (fda) has established classifications. Medical Device Product Family Definition.
From mavink.com
Medical Device Classification Chart Medical Device Product Family Definition Web organizations should develop and maintain a medical device file for each product type or device family. (1) ‘medical device’ means any. For the purposes of this regulation, the following definitions apply: Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Web manufacturers faced with testing multiple devices with common packaging. Medical Device Product Family Definition.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Product Family Definition Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. Web iso 13485 requires a medical device file for each medical device type or medical device family. For the purposes of. Medical Device Product Family Definition.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Product Family Definition Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. For the purposes of this regulation, the following definitions apply: Web organizations should develop and maintain a medical device file for. Medical Device Product Family Definition.
From perfectusbiomed.com
Supporting you on your medical device product development journey Medical Device Product Family Definition Web organizations should develop and maintain a medical device file for each product type or device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web iso 13485 requires a medical device file for each medical device type or medical device family. Web the food and drug administration (fda) has. Medical Device Product Family Definition.
From mungfali.com
Classification Of Medical Devices Medical Device Product Family Definition Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. Web organizations should develop and maintain a medical device file for each product type or device family. Web the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. (1) ‘medical device’ means. Medical Device Product Family Definition.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing Medical Device Product Family Definition Web iso 13485 requires a medical device file for each medical device type or medical device family. Web organizations should develop and maintain a medical device file for each product type or device family. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. For the purposes of this regulation, the following. Medical Device Product Family Definition.
From qbd.eu
Medical Device guide The Pathway from Idea to Patient Quality by Design Medical Device Product Family Definition Web iso 13485 requires a medical device file for each medical device type or medical device family. Web when grouping devices together to create a family of products for technical files, rather than have a technical file for. Web manufacturers faced with testing multiple devices with common packaging systems to iso 11607 can lessen their testing. Web the food and. Medical Device Product Family Definition.